Coulter Electronics (1976 - 2009)
Epics V and 750 series - 1979 through 1985
A series of instruments which were essentially 5 watt argon ion laser instruments, complete with a multiparameter data analysis system, floppy drive and graphics printer
|
EPICS V
Coulter's Original 1953 Patent application |
EPICS V Dual Laser
Hand-drawn advertising drafts of the first Coulter Counter (1956) |
|
EPICS 541
Coulter's Original 1953 Patent application |
EPICS 750
Hand-drawn advertising drafts of the first Coulter Counter (1956) |
Stuart Schlossman
- Schlossman at the Farber Institute in Boston, began to make monoclonal antibodies to white blood cell antigens in 1978. Eventually he collaborated with Ortho Diagnostics who distributed the famous "OK T4"etc., Mabs
- Coulter Immunology also acquired rights to his antibodies
History of Coulter Hematology
1953 Model A

Electronically measured cells
1968 Model S

Completely Automated CBC
1977 S Plus

Completely Automated CBC
1985 VC

Integration of flow cytometry into a hematology analyzer
2000 Gen•S Cell

- AccuFlex
- Decision Support Rules
- IntelliKinetics
- Automated Reticulocytes
- Integrated SlideMaker and SlideStainer
1985 VC
- Random Access
- AccuCount Technology
- Extended Linearity
- Decreased need for Manual Intervention
- WBC Interference Correction
- NRBC enumeration
History of Coulter Flow Cytometry
1975 TPS

Two Parameter Cell Sorting
1984 Epics C

Clinical Flow Sorter
1986 Epics PROFILE
Clinical Flow Analyzer
1987 Q Prep

Automated Sample Prep
1988 Cyto-Stat Monoclonal Antibodies

- Ready to use
- No Wash
- Single and Multicolor Antibodies
1993 EPICS XL / XL-MCL

- Bench-top analyzer
- Four color analysis
- Autoloader for samples
- Digital Signal Processing
Flow Cytometers
Modern flow cytometers are able to analyze several thousand particles every second, in "real time," and can actively separate and isolate particles having specified properties. A flow cytometer is similar to a microscope, except that, instead of producing an image of the cell, flow cytometry offers "high-throughput" (for a large number of cells) automated quantification of set parameters. To analyze solid tissues, single-cell suspension must first be prepared.
Early flow cytometers were, in general, experimental devices, but recent technological advances have created a considerable market for the instrumentation, as well as the reagents used in analysis, such as fluorescently-labeled antibodies and analysis software. Modern instruments usually have multiple lasers and fluorescence detectors (the current record for a commercial instrument is 4 lasers and 18 fluorescence detectors).
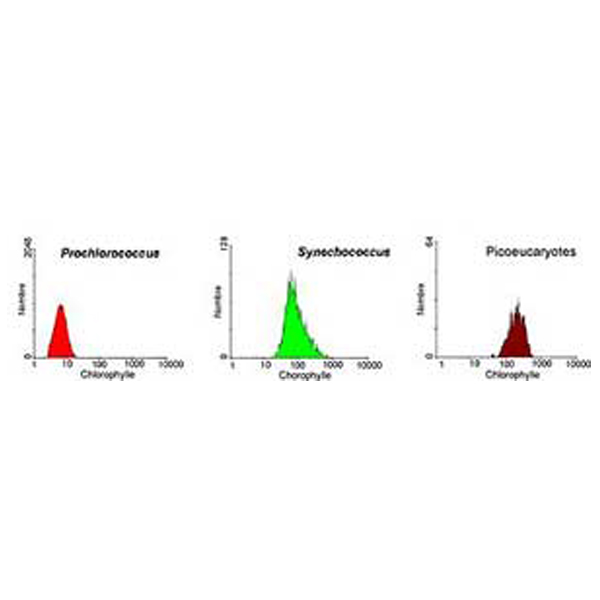 |
Analysis of a marine sample of photosynthetic picoplankton by flow cytometry showing three different populations (Prochlorococcus, Synechococcus, and picoeukaryotes)
Increasing the number of lasers and detectors allows for multiple antibody labeling, and can more precisely identify a target population by their phenotype. Certain instruments can even take digital images of individual cells, allowing for the analysis of fluorescent signal location within or on the surface of cells. The data generated by flow-cytometers can be plotted in a single dimension, to produce a histogram, or in two-dimensional dot plots or even in three dimensions. The regions on these plots can be sequentially separated, based on fluorescence intensity, by creating a series of subset extractions, termed "gates." Specific gating protocols exist for diagnostic and clinical purposes especially in relation to hematology. The plots are often made on logarithmic scales. Because different fluorescent dyes' emission spectra overlap [1], signals at the detectors have to be compensated electronically as well as computationally. Often, data accumulated using the flow cytometer can be re-analyzed (using software, e.g., Kaluza™ [5]) elsewhere, freeing up the machine for other people to use.
Commercial Instruments
Beckman Coulter's complete range of automation and information systems help you streamline processes for maximum efficiency. From delivery of more timely, accurate and reliable patient test results to the elimination of bottlenecks, our automation and information system solutions empower you to manage lab operations more efficiently and cost-effectively.





